Abstract
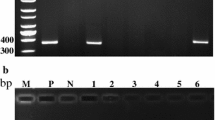
The Pineapple mealybug wilt-associated virus (PMWaV) provokes a disease that causes considerable losses to pineapple growers due to the reduced leaf tissue turgescence, resulting in leaf necrosis and death in severe cases. In this work, we describe the comparison of shoot tip culture and cryotherapy methods to eradicate the pineapple wilt disease-associated ampeloviruses. Plants from the accessions Ananas comosus var. comosus (AGB-009), var. bracteatus (AGB-119), and var. parguazensis (AGB-376) were indexed by RT-PCR, confirming mixed infections of PMWaV-1, PMWaV-2, and PMWaV-3. The accessions were cultured in vitro and their shoot tips treated by cryotherapy following a droplet vitrification protocol. The regeneration rate from shoot tip culture was 93% for AGB-376 and 100% for the other two accessions. After freezing, AGB-376 had 100% regeneration subsequent to exposure to PVS2 for 45 min, followed by 95% for AGB-009, while for AGB-119 the optimal exposure time was 60 min, with plant regeneration from nearly 80% of the shoot tips. For the accessions AGB-009 and AGB-376, all the recovered plants were virus free by the two methods, while 50% of the plants from accession AGB-119 remained infected. These results indicate that shoot tip culture alone or in association with cryotherapy is a promising routine method for virus removal from pineapple plant tissues and is useful to ensure that backup reserves of pineapple germplasm, conserved by in vitro bank and cryobank, are formed with virus-free plants.


Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Agüero J, Vives MC, Velázquez K, Ruiz-Ruiz S, Juárez J, Navarro L, Moreno P, Guerri J (2013) Citrus leaf blotch virus invades meristematic regions in Nicotiana benthamiana and citrus. Mol Plant Pathol 14:610–616. https://doi.org/10.1111/mpp.12031
Benson E (2008) Cryopreservation theory. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, Corvallis, pp 15–32
Ding F, Jin SX, Hong N, Zhong Y, Cao Q, Yi GJ, Wang GP (2008) Vitrification-cryopreservation, an efficient method for eliminating Candidatus Liberobacter asiaticus, the citrus Huanglongbing pathogen, from in vitro adult shoot tips. Plant Cell Rep 27:241–250. https://doi.org/10.1007/s00299-007-0467-8
Engelmann F, Arnao MTG, Wu U, Escobar R (2008) The development of encapsulation dehydration. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, Corvallis, pp 59–75
Faccioli G, Marani F (1998) Virus elimination by meristem tip culture and tip micrografting. In: Hadidi A, Khetarpal ARK, Koganezawa H (eds) Plant virus diseases control. American Phytopathological Society, St. Paul, pp 346–380
Faostat, Food and Agriculture Organization of the United Nations (2020) Agricultural production. URL http://faostat.fao.org/site/339/ default.aspx. Cited 26 Feb 2020
Gambino G, Perrone I, Gribaudo I (2008) Rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem Anal 19:520–525. https://doi.org/10.1002/pca.1078
Gambley CF, Steele V, Geering ADW, Homas JE (2008) The genetic diversity of ampeloviruses in Australian pineapples and their association with mealybug wilt disease. Aust Plant Pathol 37:95–105. https://doi.org/10.1071/AP07096
Kushnarenko S, Romadanova N, Aralbayeva M, Zholamanova S, Alexandrova A, Karpova O (2017) Combined ribavirin treatment and cryotherapy for efficient Potato virus M and Potato virus S eradication in potato (Solanum tuberosum L.) in vitro shoots. In Vitro Cell Dev Biol – Plant 53:425–432. https://doi.org/10.1007/s11627-017-9839-0
Li BQ, Geng CH, Hu LY, Wang MR, Wang QC (2016) Shoot tip culture and cryopreservation for eradication of Apple stem pitting virus (ASPV) and Apple stem grooving virus (ASGV) from apple rootstocks ‘M9’ and ‘M26’. Ann Appl Biol 168:142–150. https://doi.org/10.1111/aab.12250
Murashige T, Skoog FA (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–492. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Plopa C, Preda S (2013) Elimination of Apple mosaic virus by tissue culture of some infected apple cultivars. Acta Hortic 981:517–522. https://doi.org/10.17660/ActaHortic.2013.981.83
Pradhan S, Regmi T, Ranjit M, Pant B (2016) Production of virus-free orchid Cymbidium aloifolium (L.) Sw. by various tissue culture techniques. Heliyon 2:e00176. https://doi.org/10.1016/j.heliyon.2016.e00176
Retheesh ST, Bhat AI (2010) Simultaneous elimination of Cucumber mosaic virus and Cymbidium mosaic vírus infecting Vanilla planifolia through meristem culture. Crop Prot 29:1214–1217. https://doi.org/10.1016/j.cropro.2010.05.017
Sakai A, Hirai D, Niino T (2008) Development of PVS based vitrification and encapsulationvitrification protocols. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, NY, pp 33–57. https://doi.org/10.1007/978-0-387-72276-4_3
Sether DM, Hu JS (2002) Closterovirus infection and mealybug exposure are necessary for the development of mealybug wilt of pineapple disease. Phytopathology 92:928–935. https://doi.org/10.1094/PHYTO.2002.92.9.928
Sether DM, Karasev AV, Okumura C, Arakawa C, Zee F, Kislan M, Busto J, Hu JS (2001) Differentiation, distribution, and elimination of two different pineapple mealybug wilt-associated viruses found in pineapple. Plant Dis 85:856–864. https://doi.org/10.1094/PDIS.2001.85.8.856
Sether DM, Melzer MJ, Busto J, Zee F, Hu JS (2005) Diversity and mealybug transmissibility of ampeloviruses in pineapple. Plant Dis 89:450–456. https://doi.org/10.1094/PD-89-0450
Shin JH, Kang DK, Sohn JK (2013) Production of yam mosaic virus (YMV)-free Dioscorea opposita plants by cryotherapy of shoot-tips. CryoLetters 34:149–157
Silva RL, Ferreira CF, Ledo CAS, Souza EH, Silva PH, Costa MAPC, Souza FVD (2016) Viability and genetic stability of pineapple germplasm after 10 years of in vitro conservation. Plant Cell Tiss Org Cult 127:123–133. https://doi.org/10.1007/s11240-016-1035-0
Souza EH, Souza FVD, Junior DS, Santos-Serejo JA, Amorin EP, Ledo CAS (2012) Genetic variation of the Ananas genus with ornamental potential. Genet Resour Crop Evol 59:1357–1476. https://doi.org/10.1007/s10722-011-9763-9
Souza FVD, Kaya E, Vieira LJ, Souza EH, Amorim VBO, Skogerboe D, Matsumoto T, Alves AAC, Ledo CAS, Jenderek MM (2016) Droplet-vitrification and morphohistological studies of cryopreserved shoottips of cultivated and wild pineapple genotypes. Plant Cell Tiss Org Cult 124:351–360. https://doi.org/10.1007/s11240-015-0899-8
Tavazza R, Lucioli A, Benelli C, Giorgi D, Aloisio ED, Papacchioli V (2013) Cryopreservation in artichoke: towards a phytosanitary qualified germplasm collection. Ann Appl Biol 163:231–241. https://doi.org/10.1111/aab.12049
Wang B, Wang R-R, Cui Z-H, Bi WL, Li J-W, Li B-Q, Ozudogru AE, Volk GM, Wang Q-C (2014) Potential applications of cryogenic technologies to plant genetic improvement and pathogen eradication. Biotechnol Adv 32:583–395. https://doi.org/10.1016/j.biotechadv.2014.03.003
Wang M, Lambardi M, Engelmann F, Pathirana R, Panis B, Volk GM, Wang Q-C (2020) Advances in cryopreservation of in vitro-derived propagules: technologies and explant sources. Plant Cell Tiss Org Cult https://doi.org/10.1007/s11240-020-01770-0
Wang M-R, Li B-Q, Feng C-H, Wang Q-C (2016) Culture of shoot tips from adventitious shoots can eradicate apple stem pitting virus but fails in apple grooving virus. Plant Cell Tiss Org Cult 125:283–291. https://doi.org/10.1007/s11240-016-0948-y
Wang Q, Valkonen JPT (2009) Cryotherapy of shoot tips: novel pathogen eradication method. Trends Plant Sci 14:119–122. https://doi.org/10.1016/j.tplants.2008.11.010
Wang QC, Mawassi M, Li P, Gafny R, Tanne E (2003) Elimination of Grapevine virus A (GVA) by cryopreservation of in vitro-grown shoot tips of Vitis vinifera L. Plant Sci 165:321–327. https://doi.org/10.1016/S0168-9452(03)00091-8
Wang QC, Valkonen JPT (2008) Elimination of two viruses which interact synergistically from sweetpotato by shoot tip culture and cryotherapy. J Virol Methods 154:135–145. https://doi.org/10.1016/j.jviromet.2008.08.006
Wang RR, Mou HQ, Gao XX, Chen L, Li MF, Wang QC (2015) Cryopreservation for eradication of Jujube witches’ broom phytoplasma from Chinese jujube (Ziziphus jujuba). Ann Appl Biol 166:218–228. https://doi.org/10.1111/aab.12175
Acknowledgments
We thank Embrapa-CNPMF for the infrastructure and technical support.
Funding
This work received support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (407136-2016-9 and 304269/2018-2), Fundação de Amparo a Pesquisa do Estado da Bahia (APP0040/2016), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES 001, CAPES/ Embrapa, UFRB/PNPD - 88882.315208/2019-01, PROCAD - 88887.124186/2014-00) through the scholarships granted.
Author information
Authors and Affiliations
Contributions
PAG, DASM, and RSO contribute to acquisition, analysis, and interpretation of data. EHS and ECA contributed to analysis and interpretation of data and writing and revision of the manuscript. FVDS contributed to conception and design of the experiment and to writing and revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Confict of interest
The authors declare they have no conflicts of interest.
Additional information
Editor: Barbara Reed
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guerra, P.A., de Souza, E.H., de Andrade, E.C. et al. Comparison of shoot tip culture and cryotherapy for eradication of ampeloviruses associated with Pineapple mealybug wilt in wild varieties. In Vitro Cell.Dev.Biol.-Plant 56, 903–910 (2020). https://doi.org/10.1007/s11627-020-10100-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-020-10100-0